 |
 |
| Korean J Ophthalmol > Volume 31(1); 2017 > Article |
Abstract
Purpose
To investigate the relationships between tear osmolarity and other ocular surface parameters and to determine the diagnostic value of tear osmolarity in primary Sj├Čgren's syndrome (SS) using tear film break-up time, Schirmer I test, and cornea/conjunctiva staining.
Methods
We included 310 eyes of 155 patients diagnosed with dry eye disease (39 primary SS and 116 non-Sj├Čgren dry eye disease) at Seoul St. Mary's Hospital from August 2010 to January 2015. All subjects completed the Ocular Surface Disease Index (OSDI) questionnaire and underwent ocular examinations including tear osmolarity (TearLab Osmolarity System), Schirmer I test, slit lamp examination for tear film break-up time, and corneal and conjunctival fluorescein staining. We used the mean value of both eyes for all parameters. Fluorescein staining was assessed using the Sj├Čgren's International Collaborative Clinical Alliance ocular staining score (OSS).
Results
In primary SS patients (n = 39), the mean subject age was 52.5 ┬▒ 11.9 years, and 94.9% of the subjects were women. Mean tear osmolarity in SS was 311.1 ┬▒ 16.4 mOsm/L, with 16 (41.0%) subjects having values Ōēź316 mOsm/L. In SS, there was a positive correlation between mean tear osmolarity and OSDI score (Žü = 0.405, p = 0.011) and OSS (Žü = 0.592, p < 0.001). There was a negative correlation between mean tear osmolarity and the Schirmer I test (Žü = ŌłÆ0.625, p < 0.001). There was no significant correlation between mean tear osmolarity and tear film break-up time in SS (Žü = 0.110, p = 0.505).
Sj├Čgren's syndrome (SS) is a chronic systemic autoimmune disease affecting mainly middle-aged women [1]. It has a wide clinical spectrum that extends from sicca symptoms on the mucosal surfaces to extraglandular manifestations [2]. Lymphocytic infiltration and destruction of exocrine glands (mainly the salivary and lacrimal glands) is the histologic hallmark of SS, resulting in dry eye and dry mouth [3]. In addition, systemic features of a cutaneous, respiratory, renal, hepatic, neurologic, and vascular nature often occur [2,4,5]. Primary SS occurs alone, while secondary SS occurs in conjunction with another autoimmune disease [6,7]. Occasionally, ocular symptoms precede other features, but the diagnosis of SS is often delayed owing to its ambiguous symptoms. In one study [8], time from the first symptom to a diagnosis of SS was a mean of 7 years, with considerable psychological distress caused by unexplained symptoms during that time. Therefore, early non-invasive diagnosis is necessary for SS.
Several tools are used to evaluate the severity of dry eye disease, including tear film break-up time, fluorescein or lissamine green staining of the cornea and conjunctiva, and the Schirmer test, but these tools are limited by their low specificity and interobserver variation [9]. Moreover, sometimes these tools have a low correlation with subjective symptoms. Much effort has been made to find an ideal diagnostic tool that is simple, specific, sensitive, objective, non-invasive, reproducible, and has a clear cut-off value. In recent years, tear osmolarity has been considered a reliable diagnostic marker of dry eye disease [10,11,12,13]. Further, technology has become available to measure tear osmolarity in the office setting with a microfluidic lab-on-a-chip device based on electrical impedance [14]. The TearLab Osmolarity System (TearLab, San Diego, CA, USA) can collect a tear sample atraumatically and provides an absolute numeric score. It provides results in a minute, and there is no interobserver variation.
Data on the correlation between tear osmolarity and other dry eye severity markers is conflicting in the literature. Recent studies show that tear osmolarity has a correlation with the severity of dry eye in rheumatoid arthritis [15], ocular mucous membrane pemphigoid [16], and ocular graft-versus-host disease [17]. However, Amparo et al. [18] reported that changes in tear osmolarity did not contribute to prediction of dry eye symptoms or corneal staining after treatment. Further, other authors have reported that the applicability of TearLab is restricted by low reproducibility and wide variation in repeat measurements [12,19,20].
In this study, we focused on the correlations between tear osmolarity and other ocular surface parameters to further analyze the diagnostic value of tear osmolarity in SS. Bunya et al. [21] have already investigated the relationship between tear osmolarity, the Schirmer I test, and dry eye symptoms in SS. However, their study did not include currently available tools, such as tear film break-up time and cornea/conjunctiva staining. Therefore, we included these parameters in our study.
This cross-sectional study comprised 310 eyes in 155 patients. We enrolled 39 patients diagnosed as having primary SS and 116 with non-Sj├Čgren dry eye disease who were matched for age and sex. Inclusion criteria were age over 20 years and a proven diagnosis of dry eye disease at Seoul St. Mary's Hospital from August 2010 to January 2015. SS was diagnosed according to the proposed international criteria by American-European Consensus Group (2002) [22] and confirmed by both an ophthalmologist and a rheumatologist.
Exclusion criteria included active ocular inflammation or infection not associated with dry eye, drug toxicity, a history of ocular surgery or trauma, and wearing of contact lenses. Subjects using systemic medications that could interfere with tear production such as diuretics, beta-blockers, benzodiazepines, and antihistamines were excluded, but patients taking systemic immunosuppressive agents for SS were included. Subjects who had used any eye drops in the 6 hours before the study were also excluded.
The study design followed the standards for biomedical research laid down in the Declaration of Helsinki, and the protocols used were approved by the institutional review board of the Catholic University of Korea.
Demographic information, current systemic or topical treatments, and other medical history were recorded. Ocular examinations including visual acuity, non-contact pneumatic tonometry, and anterior segment slit lamp examination were performed for all subjects. The tests for dry eye were performed in the following order: Ocular Surface Disease Index (OSDI) questionnaire, tear osmolarity, Schirmer I test, slit lamp examination for tear film break-up time, and corneal and conjunctival fluorescein staining. We allowed a 5-minute interval between the tests.
We assessed subjective symptoms using the OSDI questionnaire, which included 12 questions regarding dry eye symptoms during the past week; each symptom was graded from 0 to 4, for a final score of 0 (mild) to 100 (severe). A higher OSDI score represents greater disability.
Tear osmolarity was evaluated using the TearLab Osmolarity System. A 50-nL tear sample was collected from the inferior lateral meniscus of each eye, and the mean value of both eyes was used for the statistical analysis. Tear osmolarity Ōēź316 mOsm/L has been reported to identify dry eye disease with high sensitivity, specificity, and predictive accuracy [11].
A 5-minute Schirmer I test (without anesthesia) was performed before instillation of any eye drops. The standardized Schirmer test strip (Eagle Vision, Memphis, TN, USA) was bent and placed at the inferior outer fornix. Each patient was instructed to keep his/her eyes closed during the test. The length of maximal wetting was measured after 5 minutes. A Schirmer test value Ōēż5 mm was considered abnormal.
To measure tear film break-up time, we placed a fluorescein-impregnated strip in the lateral part of inferior fornix. Each patient was asked to blink, and the time before the corneal dry spot appeared in the stained tear film was recorded as the tear film break-up time. Tear film break-up time Ōēż5 seconds was considered abnormal.
Corneal and conjunctival staining was evaluated after fluorescein staining under a yellow-barrier filter and cobalt blue illumination. Corneal and conjunctival staining was graded according to the Sj├Čgren's International Collaborative Clinical Alliance ocular staining score (OSS) [23].
All statistical analyses were performed using SPSS for Windows ver. 19.0 (IBM Co., Armonk, NY, USA). We used the mean value of both eyes for all parameters. The Mann-Whitney test was used to compare parameters between the two groups. The Spearman rank-order correlation test was used to evaluate the relationships between variables in the patients with Sj├Čgren dry eye disease. A p-value <0.05 was considered to be statistically significant in all analyses.
Table 1 shows the clinical characteristics of the 155 study participants. Their mean age was 52.7 ┬▒ 12.0 years, and 148 (95.5%) were female. Table 1 summarizes the mean (┬▒standard deviation) values for the ocular surface parameters, i.e., OSDI score, tear film break-up time, Schirmer I test, tear osmolarity, and OSS. In the Sj├Čgren dry eye group (n = 39), mean tear osmolarity in SS was 311.1 ┬▒ 16.4 mOsm/L, and 16 measurements (41.0%) were over 316 mOsm/L. Mean tear film break-up time was 3.0 ┬▒ 2.1 seconds, and 35 measures (89.7%) were Ōēż5 mm. The mean Schirmer I test value was 5.4 ┬▒ 6.9, and 24 measures (61.5%) were Ōēż5 mm, indicating significant dry eye. In the non-Sj├Čgren dry eye group (n = 116), mean tear osmolarity was 297.7 ┬▒ 12.7 mOsm/L, and 11 measures (9.5%) were over 316 mOsm/L. Mean tear film break-up time was 4.6 ┬▒ 1.9 seconds, and 76 measures (65.5%) were Ōēż5 mm. The mean Schirmer I test value was 6.7 ┬▒ 6.5, and 61 measures (52.6%) were Ōēż5 mm. Tear osmolarity value and OSS were higher in the Sj├Čgren dry eye group. In contrast, the tear film break-up time value was higher in the non-Sj├Čgren dry eye group.
The scatter plots in Fig. 1 show correlations between tear osmolarity and other ocular surface parameters in the Sj├Čgren dry eye group. There was a statistically significant positive correlation between mean tear osmolarity and OSDI score (Žü = 0.405, p = 0.011) and OSS (Žü = 0.592, p < 0.001). There was also a statistically significant negative correlation between mean tear osmolarity and the Schirmer I test (Žü = ŌłÆ0.625, p < 0.001). However, there was no significant correlation between mean tear osmolarity and tear film break-up time (Žü = 0.110, p = 0.505).
In the non-Sj├Čgren dry eye group, there were no significant correlation between mean tear osmolarity and OSDI score (Žü = 0.101, p = 0.279), Schirmer I test (Žü = 0.119, p = 0.205), tear film break-up time (Žü = 0.133, p = 0.165), or OSS (Žü = 0.145, p = 0.119).
The dry eye disease associated with SS is multifactorial in nature but is mainly aqueous deficient-type. In 2002, a revised international classification of SS was established by the American-European Consensus Group [22]. The criteria used include two objective measures of ocular involvement, i.e., a Schirmer I test result Ōēż5 mm in 5 minutes and a Rose Bengal or other ocular dye score Ōēź4 according to van Bijsterveld's scoring system. The Schirmer I test represents the tear production rate and remains the mainstay of diagnosis. However, this test has some drawbacks with regard to interpretation of results in the event of reflex tearing, and the test strip induces irritation in patients with dry eye. Aside from the Schirmer I test, tear film osmometers are becoming popular for diagnosing dry eye. Until recently, quantification of inflammatory cytokines was limited to the research setting because of logistic problems such as the need for laboratory equipment and evaporation of tear samples in transit. In 2009, the TearLab Osmolarity System was approved by the U.S. Food and Drug Administration for assessment of dry eye. This system has several strengths over other clinical tools. The purpose of our study was to identify correlations between tear osmolarity and other ocular surface parameters in SS.
Comparing our Sj├Čgren dry eye and non-Sj├Čgren dry eye groups, tear film break-up time ( p < 0.001) and Schirmer I test (p = 0.052, no statistical significance) turned out to be worse in the Sj├Čgren dry eye group. These results suggest that dry eye associated with SS is generally more severe than in non-Sj├Čgren dry eye.
In our patient population with SS, there was a positive correlation between tear osmolarity and OSDI score and OSS and a negative correlation between tear osmolarity and the results of the Schirmer I test. This finding is con-sistent with the results reported by Utine et al. [24] who found a positive correlation between tear osmolarity and OSDI score and a negative correlation between tear osmolarity and the results of the Schirmer test with anesthesia. In contrast, Bunya et al. [21] found that a higher tear osmolarity was associated with lower scores on the OSDI questionnaire in SS patients. They hypothesized that the worse was the dry eye disease, the less sensitive was the cornea. In our patient population, the OSDI score was not significantly affected (mean, 23.4 ┬▒ 18.9). These results suggest that the OSDI score is not necessarily proportional to the severity of dry eye disease [25,26].
Interestingly, we found no correlation between tear osmolarity and tear film break-up time in SS patients. One possible explanation for this is the subjective nature of the tear film break-up time test. Another explanation is that tear film hyperosmolarity in SS can be mainly induced by increased release of inflammatory cytokines into the tear film instead of a decreased tear volume because osmolarity is defined as the amount of osmotically active solutes (osmoles) present in 1 L of solution [27]. Further, an impact on this result is incidental because, as shown in the revised international classification for Sj├Čgren's syndrome (2002), the Schirmer test and corneal staining score are more important than tear film break-up time when diagnosing SS.
Tomlinson et al. [11] undertook a meta-analysis to determine the cut-off value for diagnosing dry eye disease; they proposed a cut-off of Ōēź316 mOsm/L (with a sensitivity of 69% and specificity of 92%) for diagnosis of dry eye. In contrast, Szalai et al. [28] and Messmer et al. [29] have demonstrated that tear film osmolarity lacks the ability to distinguish between patients with and without dry eye. In our study population, mean tear osmolarity in the patients with SS was 311.1 ┬▒ 16.4 mOsm/L; in 16 patients (41%), this value was over 316 mOsm/L. To the best of our knowledge, there is no suggested cut-off value for SS-related dry eye disease. Tear osmolarity can be affected by a number of environmental factors, including air flow, humidity, the patient's general state of health, systemic medication, and seasonal or diurnal variation. Further, some authors have shown that a tear sample taken from the meniscus may underestimate tears over the surface of the eye, especially in patients with dry eye disease [30]. Further investigations should be conducted to establish a cut-off value in consideration of the various factors affecting tear osmolarity.
Our study has some limitations. First, the results may be confounded by a lack of data on relevant variables, such as systemic medications and the effects of environmental factors on measurements. Although the effect of systemic medication on tear film stability remains unclear, a previous study concluded that systemic anti-inf lammatory treatment did not significantly affect tear osmolarity or OSDI score in patients with SS [21]. Second, the current study did not evaluate repeat measurements in the participants. Bunya et al. [20] found a high degree of variability for tear osmolarity measured by the TearLab Osmolarity System; there was an error associated with repeat mea-surements during one session in the patients with SS. Third, we found no correlation between tear osmolarity and other ocular surface parameters in non-Sj├Čgren dry eye group. We suppose the reason is that non-Sj├Čgren dry eye is multifactorial disease, although additional study is needed to answer this question. Finally, the present study was limited by the lack of inclusion of a control (non-dry eye disease) cohort, so it was not possible to evaluate normal variations. However, a major strength is that we evaluated almost all of the conventional tests for dry eye disease.
In summary, our study demonstrates that tear osmolarity can reflect both symptom severity (OSDI score) and objective signs such as the Schirmer test and OSS. In clinical practice, tear osmolarity can have a central role in screening and diagnosing dry eye disease in patients with SS. Further large-scale investigations should be performed to evaluate whether tear osmolarity is of value in tracking the therapeutic response and for setting a cut-off value specific for SS-related dry eye disease.
Notes
This study was presented as a narration at the 115th annual meeting of the Korean Ophthalmological Society in Busan, Korea, in 2016.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Moutsopoulos HM, Tzioufas AG. Sjogren's syndrome. In: Kasper DL, Fauci AS, Hauser S, , Harrison's principles of internal medicine. 19th ed. New York: Mc-Graw Hill Education Medical; 2015. p. 383.
2. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med 2004;164:1275-1284.


3. Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren's syndrome. Nat Rev Rheumatol 2010;6:529-537.


4. Mavragani CP, Moutsopoulos NM, Moutsopoulos HM. The management of Sjogren's syndrome. Nat Clin Pract Rheumatol 2006;2:252-261.

5. Reina D, Roig Vilaseca D, Torrente-Segarra V, et al. Sjogren's syndrome-associated interstitial lung disease: a multicenter study. Reumatol Clin 2016;12:201-205.


6. Fox PC. Autoimmune diseases and Sjogren's syndrome: an autoimmune exocrinopathy. Ann N Y Acad Sci 2007;1098:15-21.


7. Dafni UG, Tzioufas AG, Staikos P, et al. Prevalence of Sjogren's syndrome in a closed rural community. Ann Rheum Dis 1997;56:521-525.



8. Segal B, Bowman SJ, Fox PC, et al. Primary Sjogren's syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes 2009;7:46



9. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:179-193.


10. Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea 2012;31:1000-1008.


11. Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci 2006;47:4309-4315.


12. Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci 2010;51:6125-6130.


13. Khanal S, Tomlinson A, McFadyen A, et al. Dry eye diagnosis. Invest Ophthalmol Vis Sci 2008;49:1407-1414.


14. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 2011;151:792-798.e1.


15. Schargus M, Wolf F, Tony HP, et al. Correlation between tear film osmolarity, dry eye disease, and rheumatoid arthritis. Cornea 2014;33:1257-1261.


16. Miserocchi E, Iuliano L, Berchicci L, et al. Tear film osmolarity in ocular mucous membrane pemphigoid. Cornea 2014;33:668-672.


17. Na KS, Yoo YS, Hwang KY, et al. Tear osmolarity and ocular surface parameters as diagnostic markers of ocular graft-versus-host disease. Am J Ophthalmol 2015;160:143-149.e1.


18. Amparo F, Jin Y, Hamrah P, et al. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol 2014;157:69-77.e2.


19. Khanal S, Millar TJ. Barriers to clinical uptake of tear osmolarity measurements. Br J Ophthalmol 2012;96:341-344.


20. Bunya VY, Fuerst NM, Pistilli M, et al. Variability of tear osmolarity in patients with dry eye. JAMA Ophthalmol 2015;133:662-667.



21. Bunya VY, Langelier N, Chen S, et al. Tear osmolarity in Sjogren syndrome. Cornea 2013;32:922-927.



22. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554-558.



23. Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren's Syndrome International Registry. Am J Ophthalmol 2010;149:405-415.


24. Utine CA, Bicakcigil M, Yavuz S, Ciftci F. Tear osmolarity measurements in dry eye related to primary Sjogren's syndrome. Curr Eye Res 2011;36:683-690.


25. Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf 2009;7:28-40.


26. Dastjerdi MH, Dana R. Corneal nerve alterations in dry eye-associated ocular surface disease. Int Ophthalmol Clin 2009;49:11-20.


27. Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf 2005;3:81-95.


28. Szalai E, Berta A, Szekanecz Z, et al. Evaluation of tear osmolarity in non-Sjogren and Sjogren syndrome dry eye patients with the TearLab system. Cornea 2012;31:867-871.


Fig.┬Ā1
Scatter plot showing significant correlations between Ocular Surface Disease Index (OSDI) score and average tear film osmolarity (Žü = 0.405, p = 0.011), average Schirmer I test and average tear film osmolarity (Žü = ŌłÆ0.625, p < 0.001), and average ocular staining score (OSS) and average tear film osmolarity (Žü = 0.592, p < 0.001). The Spearman rank-order correlation test was used to evaluate the relationship between variables. OSS was by Sj├Čgren's International Collaborative Clinical Alliance [23].

Table┬Ā1
Clinical characteristics of study participants
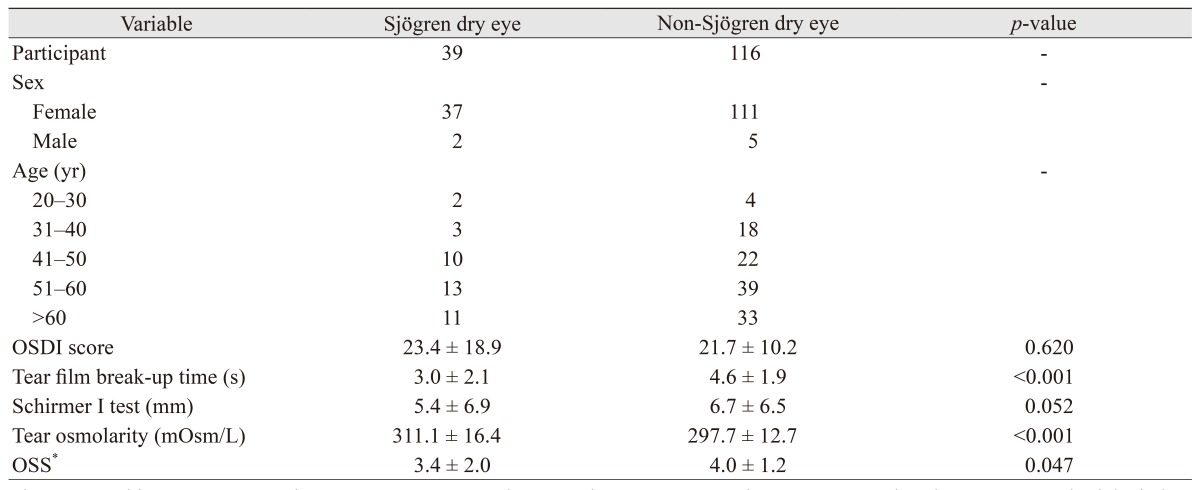
The Mann-Whitney test was used to compare parameters between the two groups. Values are presented as the mean ┬▒ standard deviation.
OSDI = Ocular Surface Disease Index; OSS = ocular staining score.
*By Sj├Čgren's International Collaborative Clinical Alliance [23].
- TOOLS
-
METRICS

- Related articles
-
The Correlation between Angle Kappa and Ocular Biometry in Koreans2013 December;27(6)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



